how to submit applications
Reesearch work involving GMOs
FLOW CHART
The process for assessment and notification of research work involving GMOs can be found here:
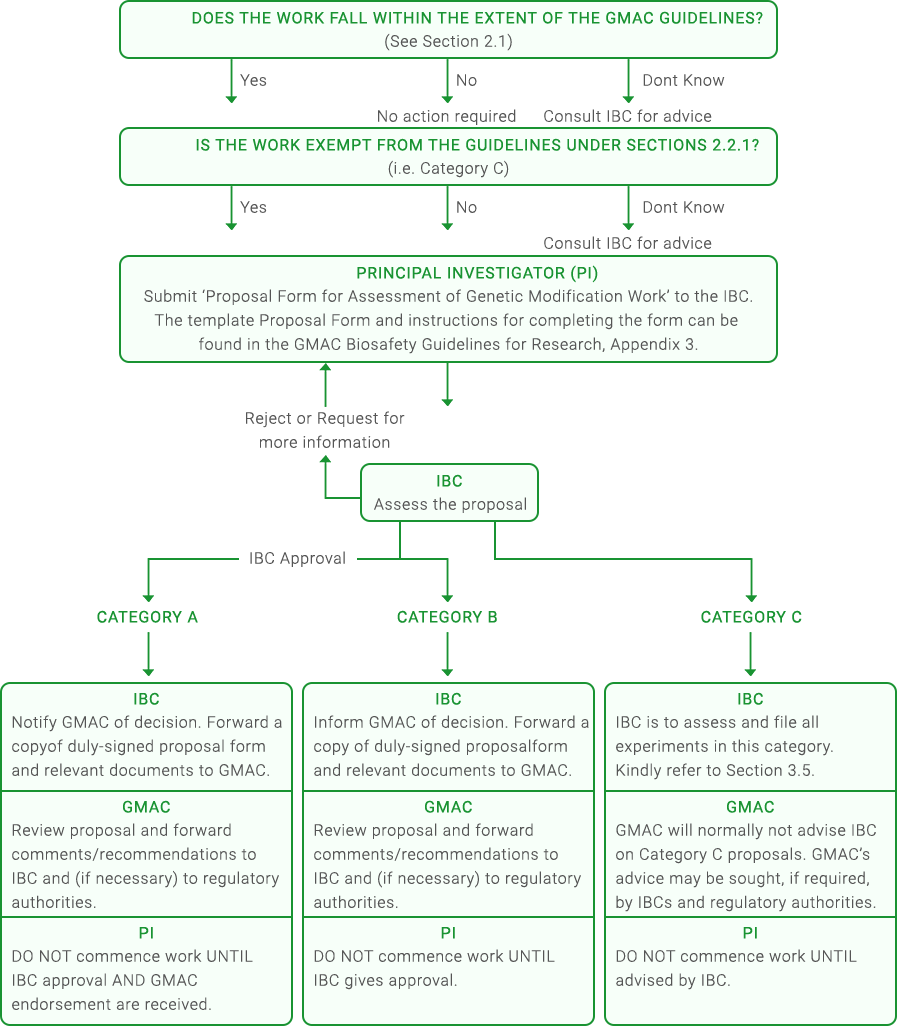
Upon categorisation of the application, please proceed to submit the application form to the GMAC secretariat at info@gmac.sg
Document(s) to download
Proposal Form for Assessment of GM Work (April 2021)
Singapore Biosafety Guidelines for Research on GMOs (revised May 2021)
more information
For list of GMAC-approved model organisms, click here.
For list of GMAC-approved host, vector systems, click here.
For Notice to Adopt the Revised Singapore Biosafety Guidelines on the Research on GMOs, click here.
Release of Agriculture-related GMOs
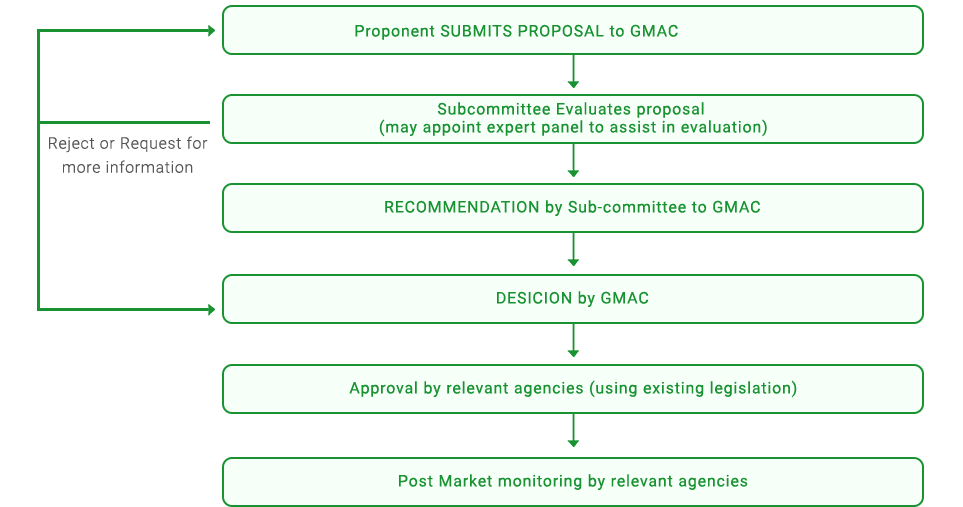
Flowchart
The process for the evaluation and approval of agriculture-related GMOs in Singapore is outlined in the flowchart.

download
Download the Guidance document.

prepare a dossier for submission
Provide details of your GM product according to the Release Guidelines.

Submit
Submit your dossier to the GMAC Secretariat at info@gmac.sg
Requirements For Single events
Please refer to Appendix 1 of the Singapore Guidelines on the Release of Agriculture-Related GMOs and complete Section A: Core questions and the following sections that are relevant to your GM product.
Requirements for Stacked events
If your product consists of stacked GM events (multiple GM events), please refer to Annex A and submit relevant documents.
Document(s) to download
Singapore Guidelines on the Release of Agriculture-Related Genetically Modified Organisms (GMOs)
ANNEX A: Risk Assessment for Stacked Events (Revised 2020)
Release of GMO-related Health products
GMAC also works with regulatory agencies such as Singapore’s Health Sciences Authority (HSA) to ensure the environmental safety of proposed releases of GMO-related health products in Singapore, either through product registration or for use in clinical trials. Applicants including companies and research institutions/labs are advised to submit a dossier to GMAC for review, following the EU guidelines for environmental risk assessment (i.e. Second schedule of S.I. No. 500 2003 – Genetically Modified Organisms (Deliberate Release) Regulations 2003). It is a regulatory requirement for applicants to submit GMAC’s recommendations on environmental risk assessment for gene therapy products (including gene modified cells) as part of the dossier submission to HSA for clinical trial or product registration applications.
Document(s) to download
The Checklist for Environment Risk Assessment (ERA) can be found here:

