Guidelines
Overview
Currently, Singapore does not have a dedicated umbrella legislation specific for the regulation of GM technology and its products. However, GMAC has released two sets of guidelines covering the commercial release of agriculture-related GMOs and research on GMOs. These are, respectively, the Singapore Guidelines on the Release of Agriculture-Related Genetically Modified Organisms (GMOs) and the Singapore Biosafety Guidelines for Research on Genetically Modified Organisms (GMOs).
GMAC is a non-regulatory advisory committee and our guidelines are not legally-binding in nature. However, in implementing our guidelines, we work very closely with the Singapore Food Agency (SFA), the Ministry of Health (MOH), and other regulatory agencies.
The Ministry of Health, Ministry of Manpower, Ministry of the Environment and Water Resources, National Environment Agency, and Agri-Food and Veterinary Authority of Singapore (currently SFA and NParks), have issued a joint circular to encourage researchers, importers, and other relevant stakeholders dealing with GMOs to comply with the GMAC guidelines. Click here to view the multi-agency circular.
regulatory agencies we work with
-
Singapore Guidelines on the Release of Agriculture-Related GMOs
-
Singapore Biosafety Guidelines for Research on GMOs
Singapore Guidelines on the Release of Agriculture-Related GMOs
BACKGROUND
The Singapore Guidelines on the Release of Agriculture-Related GMOs was published by GMAC in August 1999 to ensure the safe release and use in Singapore of agriculture-related organisms that have been genetically modified.
SCOPE
“Agriculture-related organisms” refers to animals (including fish and invertebrates), plants, microorganisms and vaccines used in cultivation, farming, agronomy, husbandry and horticulture.
The Guidelines provide a common framework for:
- the assessment of risks of agriculture-related GMOs to human health and the environment;
- and the approval mechanisms for their release in Singapore
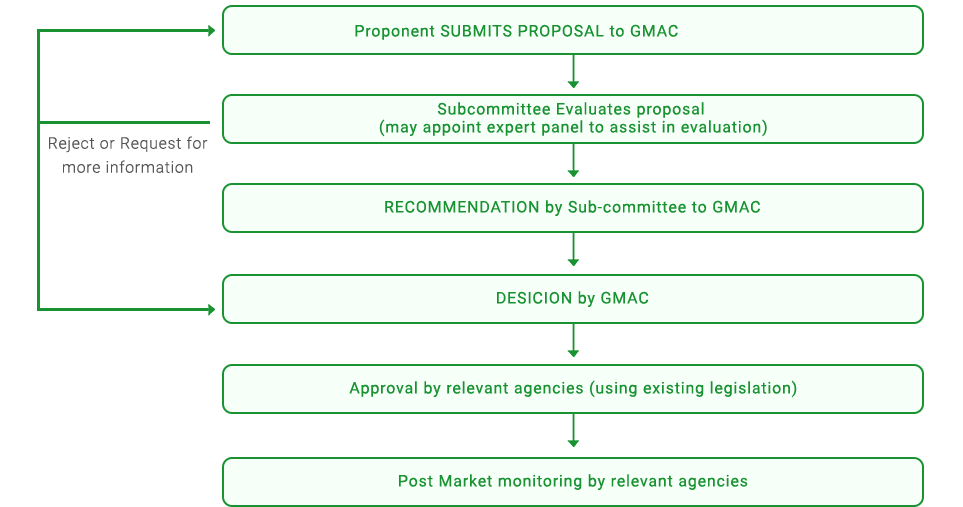
Under the Guidelines, before the release of any agriculture-related GMOs in Singapore, the proponent is required to submit a proposal to the GMAC, where the Subcommittee for the Release of GMOs and GMO-Related Products scrutinises the application in accordance to the Guidelines. For issues related to food safety, GMAC, like many other national and international agencies, adopts the concept of “substantial equivalence”. This means that if a new food or food component is found to be substantially equivalent to an existing food or food component, it can be treated to be as safe as the conventional food or food component.
Following the recommendations of the Subcommittee, GMAC will decide whether or not it will endorse the particular application. Its decisions will be forwarded to the regulatory authorities, who will take into account, among other things, GMAC’s recommendations when considering the final approval of the application.
HOW TO SUBMIT AN APPLICATION FOR REVIEW
- Download the Guidance document
- Answer Section A: Core Questions in Appendix 1, and the following questionnaire relevant to your GM product in Appendix 1.
- Submit your dossier to the GMAC Secretariat at info@gmac.sg
If your product consists of stacked GM events (multiple GM events), please refer to Annex A and submit relevant documents.
In a bid to save the environment, GMAC will no longer receive hard-copy dossier applications as of August 2018. Please kindly forward applications to the Secretariat via email.
The process for the evaluation and approval of agriculture-related GMOs in Singapore is outlined in the flowchart.
More Information
Singapore Guidelines on the Release of Agriculture-Related Genetically Modified Organisms (GMOs)
Appendix 1
Questionaire for Risk Assessment of Genetically Modified Organisms (GMOs) Related to Agriculture
Appendix 2
Risk Assessment Criteria
Annex A
Risk Assessment for Stacked Events (Revised 2020)
In a bid to save the environment, GMAC will no longer receive hard-copy dossier applications as of August 2018. Please kindly forward applications via secured file sharing platforms.
For FAQs on Release Applications, click here.
For list of events approved in Singapore, click here.
Singapore Biosafety Guidelines for Research on GMOs
BACKGROUND
In May 2006, GMAC released the Singapore Biosafety Guidelines for Research on Genetically Modified Organisms (GMOs). The Guidelines were adapted from other national and international guidelines, regulations and publications, after consultations with relevant local stakeholders such as researchers, biosafety officers, and the regulatory authorities.
The Guidelines were subsequently revised according to the relevant developments within the local and international context in Aug 2008, Jan 2013 and Jul 2020 respectively.
SCOPE
The Guidelines cover experiments that involve the construction and/or propagation of all biological entities which have been made by genetic manipulation and are of a novel genotype and which are unlikely to occur naturally or which could cause public health or environmental hazards. The Guidelines also have provisions for the importation of GMOs and/or GMO-derived products for research purposes.
The objectives of the Guidelines are to:
-
- Ensure the safe containment, handling and transport of GMOs used in research and
- To provide a common framework for assessment and notification of research on GMOs.
- Help to further enhance the biosafety culture amongst Singapore scientists working on GMOs, boosting the creditability of local research practices.
Under the Guidelines, the concept of risk assessment should be undertaken by every researcher. In addition, each institution performing work falling within the Guidelines should have an Institutional Biosafety Committee (IBC) to evaluate and oversee the work that is being done. GMAC should be notified or consulted for medium to high risk projects.
The Guidelines are not legally-binding on their own. However, GMAC works closely with regulatory agencies such as the Ministry of Health (MOH) and the National Parks Board (NParks) in implementing its guidelines. Projects will be cross-referenced between agencies whenever necessary.
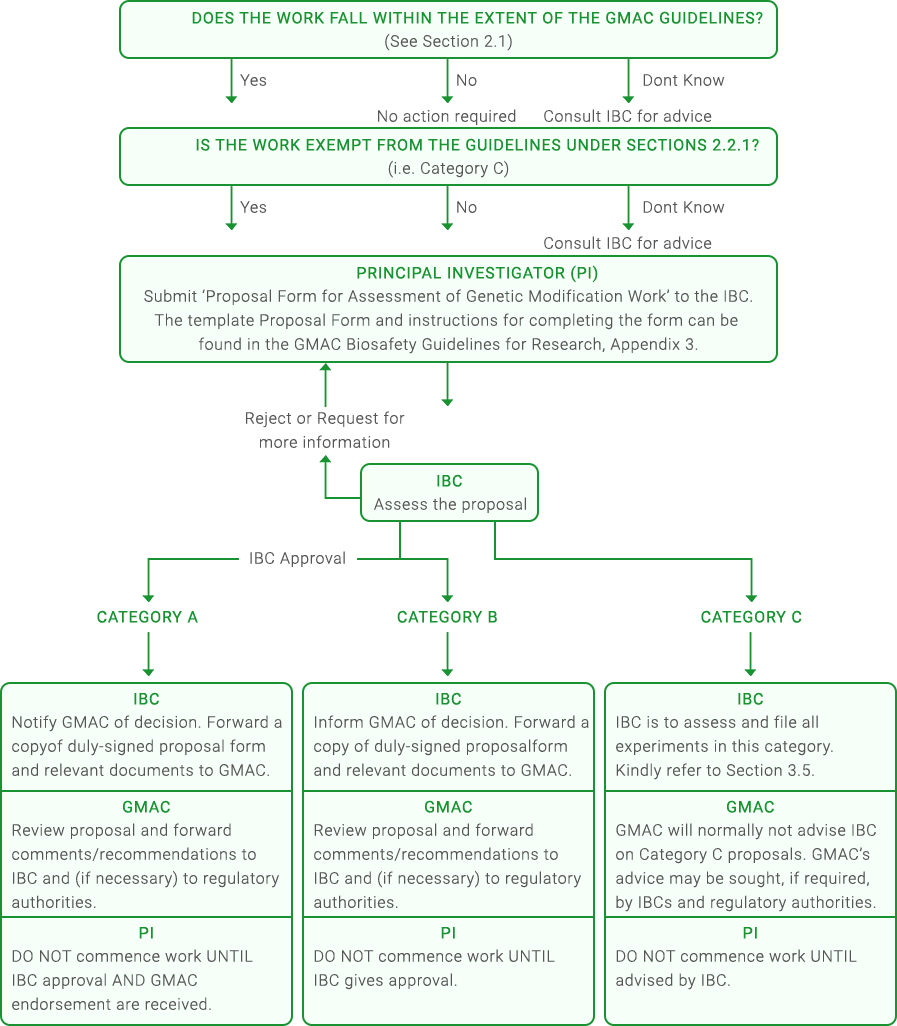
HOW TO SUBMIT AN APPLICATION FOR REVIEW
Upon categorisation of the application, please proceed to submit the application form to the GMAC secretariat at info@gmac.sg
More Information
Singapore Biosafety Guidelines for Research on GMOs (revised May 2021)
Proposal Form for Assessment of GM Work (April 2021)
[Word version available here.]
List of GMAC-approved model organisms (May 2020)
List of GMAC-approved host, vector systems (May 2021)
For FAQs on Research Proposals, click here.







