Categories
-
Ethical Considerations for Genetic Modification
-
Environment and Health Risks
-
Genetically Modified Animals
-
Genetically Modified Foods
-
Genetically Modified Microorganisms
-
Genetically Modified Organisms
-
GMOs in the Biomedical Sciences
Ethical Considerations for Genetic Modification
-
How is GM technology applicable in the biomedical sciences?
Some of the most promising and powerful applications of GM technology are in the field of biomedical sciences. Since 1982, microorganisms have been genetically modified to produce pharmaceuticals for the treatment of human diseases. More recently, through what is known as “biopharming”, scientists have explored genetically modifying plants and animals so that these become living “factories” producing pharmaceuticals. Gene therapy trials have also been used with some success for the treatment of diseases such as severe combined immunodeficiency (SCID).
-
Can you provide some examples of drugs developed from GM technology?
The first genetically engineered drug is human insulin, produced under the trade name Humulin®. Approved by the United States Food and Drug Administration (USFDA) in 1982, Humulin® is produced by genetically engineering Escherichia coli bacteria to express DNA encoding human insulin. Before genetically engineered insulin became available, diabetic patients had to rely on insulin derived from the pancreases of animals such as cows and pigs. Patients can sometimes develop allergic reactions towards insulin derived from animal sources, especially if the preparations are of low purity. GM technology has thus provided diabetic patients with a source of low-cost, reliable, and high-quality insulin.
Numerous genetically engineered drugs have been subsequently developed and approved for use. The list includes, among others, human growth hormones, Orencia® for the treatment of rheumatoid arthritis, Remicade® for the treatment of Crohn’s disease and Herceptin® for the treatment of advanced breast cancer.
In 1986, the USFDA’s approval of a genetically engineered hepatitis B vaccine marked the start of the use of genetically engineered vaccines for humans. Most recently, in May 2012, the USFDA approved the use of a drug meant to treat a disorder known as Gaucher disease, Elelyso – produced using carrot cells.
Many more genetically engineered drugs are in the pipeline.
-
What is biopharming?
Biopharming (or pharming) refers to the use of GM technology to insert genes encoding useful pharmaceuticals into host animals or plants that would otherwise not express those genes. The host animals or plants then become living “factories”, from which useful pharmaceutical products can be harvested.
Proponents believe that biopharming offers an easily controllable, safe, and cost-effective method for manufacturing pharmaceuticals. While genetically engineered drugs are most commonly produced today using microorganisms in bioreactors, pharming requires less expensive infrastructure and production can be scaled up quickly in response to demands.
In 2006, the European Commission approved the first transgenic animal-derived drug – ATryn®. Derived from the milk of GM goats, ATryn® is a recombinant form of human antithrombin and can be used therapeutically as an anticoagulant.
The USFDA approved the use of another drug in 2012. This drug which treats Gaucher disease, is named Elelyso and is produced using carrot cells. Gaucher disease is an inherited metabolic disorder that leads to liver and bone problems.
Scientists and industry players are looking forward to GM plants in developing treatments for some of the most serious diseases such as cancer, HIV, Alzheimers, diabetes, and arthritis. Ongoing developments in this area include plant biopharmaceuticals which target hepatitis C, influenza and antibiotic-associated diarrhoea.
-
What is gene therapy?
DNA is the blueprint determining the characteristics of living organisms. Genes are specific segments of DNA and each gene encodes a product with a biological effect.
Sometimes, individual genes may become defective, leading to disease manifestations. Many diseases including cystic fibrosis, severe combined immunodeficiency (SCID), thalassemia, and sickle-cell anaemia are the result of just one malfunctioning gene.
Gene therapy has the potential to treat these genetic diseases. In a nut shell, it consists of the following steps:
The gene responsible for the disease is identified
- Functional copies of the gene are made available. In principle, cells from a healthy person can be removed and the specific gene isolated. Copies of this functional gene can then be made in the laboratory.
- Target cells bearing the “faulty” gene are removed from the patient.
- A carrier (also known as the vector) is used to insert a copy of the functional gene into the DNA of target cells. Currently, the most common type of vectors are viruses. These viral vectors are genetically engineered to replace their disease-causing genes with the therapeutic genes.
- The target cells now bear two copies of the gene – the original, faulty copy, as well as the newly introduced functional copy. These target cells are reintroduced into the patient’s body.
- The newly introduced gene functions on behalf of the original, faulty one, leading to alleviation of disease symptoms.
Gene therapy holds great promise for a variety of diseases. However, the technology is currently still experimental and no human gene therapy product has been approved for routine clinical use.
-
If gene therapy is so promising, then why has it not been approved for routine clinical use?
Before the marketing of any new drug or therapy, regulators and scientists have the responsibility to carefully weigh the risks and benefits involved. Although gene therapy holds great promises, the biology involved can be very complex.
Potential risks of gene therapy include:
- Concerns with viral vectors
In gene therapy, viruses are commonly used for carry functional genes into target cells. Although these viral vectors have been genetically engineered to remove their disease-causing properties, there is concern that they may revert back to their harmful nature once inside the patient’s body.The viruses may also trigger the patient’s immune and inflammatory responses resulting in inflammation, toxicity and more severely, organ failure. In addition, as viruses can infect more than one population of cells, there is possibility of infection for other non-therapy-targeted cells in the process, causing other illness or diseases which can include cancer.
- Possibility of inducing tumours (insertional mutagenesis)
If the functional gene is inserted into a wrong place in a target cell’s DNA, it may disrupt the functions of other existing genes. Such disruptions may in turn lead to tumour formation.
Despite the initial hurdles and also failures in clinical trials some 20 years ago, gene therapeutics have seen much improvement in minimising its risks. Research carried out on cellular mechanisms especially in the immunological response of cells in various disease and cancer models has helped scientists understand the associated cellular mechanisms and how the immune system respond to virus-infected cells (in relation to cells undergoing gene therapy). As such, many risks leading from viral vector infection can potentially be minimised or even eradicated in some cases.
Recent developments in gene therapy have seen successes in the trials targeting diseases such as the hereditary haemophilia B, HIV and various cancer models.
- Concerns with viral vectors
-
The potential uses of GM technology in the biomedical sciences sound impressive. However, how safe are such applications?
The potential dangers of gene therapy have been answered.
All pharmaceuticals, whether GM-derived or not, are subjected to stringent scrutiny for efficacy and safety before they can be approved for marketing. Countless people have benefited since the first genetically engineered drug was approved in 1982.
Biopharming can present risks in the absence of appropriate measures. Gene flows (i.e. transfers of DNA from one population to another) and human errors can result in the contamination of conventional crops and accidental releases of pharm crops into the food supply. These undesirable effects can be minimized by using self-pollinating crops such as rice and flax to avoid gene flows, or non-food crops such as tobacco.
It is difficult to establish the absolute safety of any new technologies and GM technology is no exception. However, with proper regulatory safeguards, it is possible to derive benefits without compromising on safety.
-
In Singapore, which are the agencies involved in ensuring the safe use of biomedical-related GMOs?
In Singapore, pharmaceuticals, medicinal products, and clinical trials, whether GM-derived or not, are regulated by the Health Sciences Authority established under the Ministry of Health.
The agriculture sector is modest in land-scarce Singapore. It is therefore unlikely that any pharm crop will be cultivated on a large scale here. Nonetheless, should any party wish to carry out such agricultural activity, the Agri-Food & Veterinary Authority will ensure that the necessary safety measures are in place. The Singapore Guidelines on the Release on Agriculture-Related GMOs will apply and GMAC will provide advice accordingly.
-
What is genetic modification and what are genetically modified organisms?
The characteristics of living things are largely determined by DNA. Plants, animals, or bacteria whose DNA had been altered by molecular techniques are termed genetically modified organisms (GMOs).
With the advancement of biotechnology, scientists have developed special biochemical scissors and glue which enables them to “cut” and “paste” specific segments of DNA (called genes) from one living thing to another. The newly introduced DNA brings new characteristics to the resultant GMO.
Genetic modification is being applied to develop new benefits, such as creating crop plants with new traits and bacteria which produce pharmaceutical products.
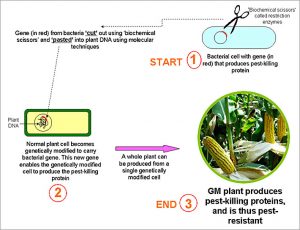
Diagram 1 below is a simple representation of how an originally pest-susceptible plant can be genetically modified to carry a bacterial gene which makes it pest-resistant.
Diagram 1: Creation of a Genetically Modified Drought-Resistant Plant
-
What are the differences between genetic modification and conventional breeding?
Both conventional breeding and genetic modification are methods for intentional manipulation of an organism’s heritable traits. To illustrate the differences, we take the example of producing drought-resistant corn.
To make an originally susceptible corn variety resistant to drought, conventional breeders can cross the susceptible corn variety with its resistant wild cousin. Offsprings exhibiting drought-resistance (i.e. the desired quality) are then selected and crossed with its resistant parent (backcrossing). The subsequent offsprings are subjected to several more generations of backcrossing and selection before a new variety of corn stably exhibiting drought-resistance can be achieved.
As conventional breeding involves the transfer of many thousands of genes randomly, the outcomes are often difficult to predict and it typically takes many years before an organism with the desired characteristics can be produced. Conventional breeding is also dependent on genetic compatibility of donor and recipient organisms. As such, breeders may not be able to cross distantly-related species, or the resultant offsprings may not be viable.
Genetic modification, on the other hand, is a more precise method. As we can see from Diagram 1, it involves the identification, isolation, and introduction of specific gene(s) from donor to recipient organisms. Genetic modification also permits the transfer of genes between totally different organisms, for example from a turnip to a cereal grain or from a bacterium to corn. The first use of this was in the 1980s, where E.coli bacterium was genetically modified to carry human insulin gene. As a result of this technology, high quality insulin can now be produced cheaply for diabetic patients.
-
What are the benefits derived from GMOs?
GMOs can be valuable in many ways. Medical, agricultural, and aesthetic applications of GM animals and microorganisms are detailed under other FAQ subsections.
The regular consumer may be more interested in knowing more about the benefits of GM foods. To date, all GM foods available commercially are derived from so-called “first generation” GM plants which have been bioengineered for agronomic benefits such as pest- and herbicide-resistance.
Proponents of pest-resistant GM crops say that the application of GM technology limits the need for spraying of pesticides and that this can have a beneficial impact on the environment.
According to ISAAA’s 2012 Review on the Global Status of Commercialized Biotech/GM Crops, more than 15 million of biotech crop farmers were small, resource-poor farmers from developing countries whose increased income from biotech crops can contribute to alleviation of their poverty.
While the “first generation” GM crops do not have direct consumer benefits, some people believe that genetic modification provides a solution to the problem of global malnutrition. Scientists have developed “second generation” GM plants with enhanced nutritional contents. Although none of these have yet been commercialized, several are in the pipeline for regulatory approval. A good example will be “Golden Rice” that is rich in pro-vitaminA. The WHO estimates that up to half a million children go blind each year because of vitamin A deficiency. With this in mind, some people see “Golden Rice” as having the potential of reducing childhood blindness in developing countries.
-
What are the authorities doing to regulate gene modification?
The Genetic Modification Advisory Committee (GMAC) was established in Singapore in April 1999 to oversee and advise on the R & D, production, use, handling and release of GMOs in Singapore, ensuring that these are done in compliance with international standards.
As a non-regulatory advisory committee, GMAC works very closely with the regulatory authorities (the Agri-Food & Veterinary Authority, Ministry of Health, NParks etc) to ensure that appropriate regulation of gene modification and its products is in place.
In this regard, GMAC has formulated and released the Singapore Guidelines on the Release of Agriculture-Related GMOs to ensure that safe movement and use of agriculture-related GMOs within Singapore is observed. A second set of guidelines – the Singapore Biosafety Guidelines for Research on GMOs, was officially launched in May 2006 to ensure the safe containment, handling and transport of genetically modified organisms used in research. The Singapoer Biosafety Guidelines for Research on GMOs was revised in 2013 to keep relevant to advancing trends in the field of biomedical research.
-
Is genetic modification more dangerous than conventional breeding?
Genetic modification can be as safe as traditional breeding. When crossing two plants using conventional breeding techniques, breeders mix thousands of genes in order to acquire one or a few genetic traits.
To remove unwanted traits, breeders spend many years back-crossing the new plant varieties repeatedly with plants whose genetic components are well known. This slowly dilutes the impact of all those unwanted genetic traits that came along with the few beneficial traits.
Genetic modification brings to traditional plant breeding the ability to move specific gene(s) instead of having to move thousands at one time.This could prevent the introduction of unwanted traits and thereby offers a more predictable, and arguably safer method to produce plants with desirable traits.
-
What are the potential risks of GMOs?
With every new emerging technology, there are potential risks. For GMOs, the potential risks include:
- The danger of unintentionally introducing allergens and other anti-nutrition factors in foods
- The likelihood of transgenes escaping from cultivated GM crops into wild relatives
- The potential for pests to evolve resistance to the toxins produced by GM crops
- The risk of these toxins affecting non-target organisms
Where legislation and regulatory institutions are in place, there are elaborate steps to precisely avoid or mitigate these risks. It is the obligation of the technology innovators (i.e. scientists), producers, and the government to assure the public of the safety of the novel foods.
GMAC has drawn up guidelines for safe production, handling, and utilization of GMOs. We work very closely with the regulatory authorities to ensure that the potential benefits of biotechnology can be realized while keeping risks to a minimum.
-
What are genetically modified (GM) microorganisms?
A GM microorganism is a virus, bacterium or yeast in which the DNA has been altered in a way that does not occur naturally.
Initial efforts in genetic modification had focused on microorganisms because of the relative simplicity of their structure, and a number of commercial processes now use GM microorganisms to produce materials in a cheap and efficient way.
-
What are the applications of GM microorganisms?
Microorganisms have been used for centuries in the manufacturing of food and feed. With the advancement of gene modification techniques, GM microorganisms have now found wide applications in the following fields:
Enzyme Production
By far, the most common application of GM microorganisms is in enzyme production. These microorganisms range from fungi like Aspergillus to bacteria like Bacillus and are usually selected by the availability of genetic tools and their capabilities to produce and/or secrete enzymes. The enzymes produced may be used in making “washing powders” (lipases), in food production, or they may have important medical uses.Production of Pharmaceuticals
GM microorganisms are also being used for the commercial production of pharmaceutical products such as insulin, interferon, human growth hormone & viral vaccines. Prior to such advances in biotechnology, the few protein drugs available were taken directly from human and animal corpses. For instance, human growth hormone was taken from human corpses and insulin required to treat diabetes was collected from slaughtered pigs. These drugs were expensive as they were available only in limited supplies. Biotechnology offers a cheap and efficient way of producing high quality drugs. More information can be found under the FAQ subsection on GMOs in Biomedical Sciences.Biodegradation/Bioremediation of Xenobiotics
There is potential in using GM microorganisms for biodegradation and/or bioremediation purposes in waste treatment or in the cleaning of contaminated land and water.Crop Production & Protection
This can be done through biological control of insects, fungal diseases, and frost damage.Extraction of Metals from Ores
Some GM microorganisms should be able to provide significant benefits in the management of the environment systems.A variety of economic, market and regulatory pressures have however limited the commercial use of GM microorganisms, particularly in bioremediation.
-
What are the potential hazards associated with GM microorganisms?
Many commercial applications of GM microorganisms are conducted in closed production facilities, that is, under ‘contained use’. For example, drugs derived from GM microorganisms are produced under such conditions. Because of the tight controls on ‘contained uses’ of GM microorganisms, these applications are very unlikely to offer threats to the environment.
However, for GM microorganisms which are developed, or being considered, to solve environmental problems (e.g. for cleaning up contamination), there may be concerns about possible risks to the environment and to human health upon their release.
Many microorganisms do not cause diseases and are normally harmless to human health. However, when a microorganism is manipulated genetically, there is a chance that the modification may increase its virulence or pathogenicity. To minimize risks, scientists who work on GM microorganisms must observe proper measures to ensure biosafety.
-
What foods are produced from GMOs? What is the approximate no. of such products in the market?
According to the International Service for the Acquisition of Agri-biotech Applications (ISAAA), the principal GM crop in 2012 was GM soybean, followed by GM maize, GM cotton, and GM canola.
ISAAA also reported that in 2012, GM crops were commercially cultivated in 28 countries. Many other countries have granted regulatory approvals for GM crops to be imported for food and feed use. Since 1996 and as at 2012, a total of 2,497 regulatory approvals have been granted worldwide for the commercial cultivation and/or food and feed use of various GM crops.
Since Singapore imports more than 90% of its food products from various parts of the world, the increasing adoption of GM crops in many countries worldwide implies that it is very likely that foods with GM-derived components are sold here.
How long have GM foods and/or food ingredients been in the market?
Chymosin is an enzyme used in cheese making. In 1990, authorities from countries such as the United Kingdom and the United States approved GM chymosin for food use. This was the first approval of a GM food ingredient. In 1994, the first GM whole food, the Flavr-Savr tomato, was released for sale in the market.
-
Are the foods produced from GMOs safe for consumption?
Foods produced from GMOs can be as safe as foods from non-GMOs. Since the first GM food was commercialized almost two decades ago, there had been no known reports of hazards resulting from the consumption of GM foods.
A report was issued in 2005 by the International Council of Science (ICSU), an organization whose membership consists of 111 national academies of science and 29 scientific unions. After comprehensive analyses of 50 science-based reviews, the ICSU concludes that “currently available genetically modified foods are safe to eat.” The World Health Organization agrees that current varieties of GM foods “are not likely to present risks for human health.”
In 2010, the European Commission published a report summarizing the results of more than 130 EU-funded projects on safety of GMOs. The projects were conducted by over 500 independent research teams over a span of more than twenty-five years. The scientific evidences led EU to conclude that “there is, as of today, no scientific evidence associating GMOs with higher risks for the environment or for food and feed safety than conventional plants and organisms.”
Closer to home, the official regulatory agency for GM foods is the Agri-Food and Veterinary Authority (AVA). As a non-regulatory advisory committee, GMAC works very closely with the AVA to ensure GM food safety.
GM foods commercially available in Singapore have undergone various lines of scrutiny for safety. Firstly, under international food practices, before a GM food can be considered for commercialization, its producer must subject it to rigorous tests on quality, allergenicity, toxicity, composition, and nutritional value. Secondly, all food products derived from GMOs must be assessed to be safe by the competent national regulatory bodies of the exporting countries.
Thirdly, in accordance to the GMAC’s Guidelines on the Release of Agriculture-Related GMOs, applications for import or release of agriculture-related GMOs in Singapore are first submitted to GMAC, where an expert scientific committee examines the GMOs’ origin, the experimental procedures used to create them, and the methods used to prove that they are safe for consumption. GMAC’s endorsement of a GM product will be one of the main factors that the AVA will take into account when considering the final approval.
In addition, AVA monitors the presence of GM foods in our market through regular testing in their laboratories. The list of GM foods available in our market is listed here.
-
Are foods derived from GMOs more nutritious?
To date, all GM foods available commercially are the so-called first generation GM foods. These have been bioengineered to express agronomic traits such as pest- and herbicide-resistance and do not differ significantly from their conventional counterparts in terms of nutritional contents.
Scientists have developed second generation GM plants with enhanced nutritional contents. While these have not been commercialized as yet, several varieties are in the pipeline for regulatory approval. Two examples will be “Golden Rice” that is rich in pro-vitaminA and soybean that contains enhanced levels of omega-3 fatty acids.
-
Are we already consuming foods derived from GMOs?
Genetically modified crops, especially soybean and corn, have been approved in many countries (see our answer to the first question above, on “What foods are produced from GMOs? What is the approximate no. of such products in the market?”). These are very likely incorporated into various processed foods. Since Singapore imports food from many parts of the world, it is possible that such foods with GM-derived components are sold here in Singapore. Like any other foods sold in Singapore, these foods have been assessed to be safe based on the AVA’s rigorous safety standards.
-
Will foods produced from GMOs carry special labels in Singapore?
There are currently no legislations and guidelines for the labelling of GM foods in Singapore. Whilst this is so, GM foods, like all other food products, must meet existing food labelling requirements to facilitate tracing and recall. The local authorities will work to ensure that GM foods commercially available in Singapore are safe for consumption, and will also continue to monitor international developments closely to ensure that Singapore’s labelling requirements are up to date.
GM food labelling, where practised, is done with the intention of providing consumers with choice, and not for food safety reasons.
-
Is enough known about gene modification in view of the fact that too little is known about the risks, particularly on the long-term health and environmental effects? Do you think we’ve marketed the GM foods too early? Is this a case of profit before safety? Should there be more conclusive findings before putting genetically modified foods on the shelves?
These are useful reminders that all new technologies, whether in food, medicine, or any other applications, may pose risks without proper checks and evaluations.
Since the first GM foods were marketed for almost two decades, there has been no reliable evidence that GM foods or crops are harmful to human health or the environment. Nonetheless, public safety is of paramount importance to GMAC and we remain vigilant on new developments in relevant fields. Our charter is not to put profit before safety, but to ensure that all risks related to these products are minimized through the establishment of a proper, science-based risk assessment framework. In this way, we will be able to reap the benefits of biotechnology while ensuring that public health and safety is well protected.
-
What are genetically modified (GM) animals?
They are animals that have had changes made to their DNA through molecular techniques. Changes in the DNA give these animals new characteristics.
Transgenic animals are genetically modified animals which have had genes from another species inserted into their DNA. The first GM animals were transgenic mice, created in 1980.
Since this time, further techniques have been developed. As well as inserting genes, it is now possible to ‘knock out’ specific genes, or to make larger-scale genetic alterations. Such animals are now generally referred to as GM animals.
-
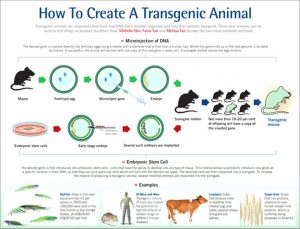
How are GM animals created?
The most common method for producing GM animals is to inject the foreign gene into fertilised eggs through a process known as ‘microinjection’.
For mammals, the injected eggs are placed into a ‘foster’ mother where they develop to term. If the foreign gene has been successfully incorporated into the egg’s original DNA, the resultant offspring will carry the extra, foreign DNA.
When this GM animal mates and produces offspring, the foreign gene is inherited in the same way as normal DNA. In this way, scientists can breed a line of GM animals that carries the extra DNA.
A diagrammatic representation of the technique is provided in Diagram 2 below.
Diagram 2: How to create a transgenic animal
(Graphics by Jeffery Lim, Copyright @ 2007 Singapore Press Holdings. All rights reserved.)
Click here for an enlarged diagram -
Which animals are used?
The first GM animal, a GM mouse, was made in the early 1980s and this technology has been successfully applied to other animals such as cattle, pigs, sheep, poultry, fish, as well as the insects such as the fruit fly (Drosophila melanogaster).
However, GM mice are still most common – they make up 98% of all GM animals.
-
What are the worst case scenarios of the damaging effects of GMOs on the environment and human population? Do we have contingency plans to cope with such events?
The most likely scenario is the accidental release of GMOs. However, the risk can be minimized significantly if proper containment measures are in place.
Before experiments on GMOs are conducted, risk assessment must be performed to ensure that such these GMOs exert minimal impact even if they should be accidentally released.
In addition, safety control measures to contain the spread of any accidentally released GMOs are in existence and these are required as pre-conditions before experiments on GMOs can be allowed to commence.
-
Why are GM animals useful?
- Medical Applications
Animal models of human diseases
Currently, this is the main medical application for GM animals.Animals may be genetically engineered to develop human diseases. For example, mice with cystic fibrosis or human cancers have been produced. By studying such animal models, scientists may gain new understandings and insights, leading to the discovery of new treatments and new drugs.Determine gene functions
Humans and certain animals such as the mice share a high degree of genetic similarity. Thus, the study of genes in GM animals may reveal how similar genes in humans work.Such use of GM animals is likely to increase significantly, because by modifying a gene, its various roles in different functional systems of the body can be identified.
Pharming for production of therapeutic proteins
Farm animals can be genetically modified to produce large quantities of useful substances for treating human patients. They become, in effect, walking pharmacies.For example, animals such as cows and goats can be genetically engineered to secrete medically valuable proteins into their milk.
Xenotransplants (transplantation from another species into humans)
Scientists have developed “Knockout pigs” by removing the specific gene that causes pig organs to be rejected by a human body.This opens the possibility of farming pigs for their organs in very much the same way that they are now farmed for pork. This can potentially alleviate the shortage of organs for organ transplants.
- Agriculture
Currently, more than thirty-five varieties of GM food fishes have been developed around the world.Some of these GM food fishes have been developed to grow faster, resist disease, and tolerate different temperatures. At least one company, Aqua Bounty Farms, is currently requesting approval from the U.S. Food and Drug Administration to market its GM salmon for food. Aqua Bounty Farms’ GM salmons can grow to more than three times larger than normal salmons within the first year of growth.Besides improving fish farm productivity, scientists have also engineered tilapia which can produce human blood clotting factors. There is potential that we can harvest these clotting factors for the treatment of human blood disorders
- Aesthetic Purposes
Professor Gong Zhiyuan of the National University of Singapore has genetically engineered zebrafish that fluoresce due to the expression of jellyfish and sea anemone genes. Trademarked Glofish®, these are the world’s first GM pets and are currently only available in the United States.
- Medical Applications
-
What are the potential hazards associated with GM animals?
Possible hazards of developing GM animals include:
- Novel or increased allergic reactions and toxic effects if they are eaten,
- Changes in behaviour of the GM animals such as increased aggression,
- Changes in the ability of the GM animal to act as a human disease reservoir,
- Impact on the ecosystem if the GM animals escaped or are released into the environment.
The likelihood of these happening is generally considered to be relatively low but certainly should not be neglected.
-
Is it ethical?
For some, genetic modification of animals is unethical because they feel that it is a form of disrespect to animals or is a violation of animal rights.
However, many others believe that an acceptable balance exists between the need to minimize animal suffering and the need to maximize gain to medicine, agriculture, and scientific understanding. Traditional breeding is also a form of genetic manipulation.
-
Can GMO production and utilization have detrimental effects on the environment?
Modern agriculture on its own, whether or not GMOs are involved, has profound impacts on all environmental resources, including negative impacts on biodiversity.
Since the commercialization of the first GM crops a decade ago, scientists have conducted extensive research on the risks that GM crops can possibly pose to the environment. In 2006, a group of researchers from Switzerland reviewed the scientific knowledge available so far and concluded that “the data available so far provide no scientific evidence that the commercial cultivation of GE crops has caused environmental harm.”
We must nonetheless be reminded that a number of issues can affect how scientific data is interpreted. Any exotic organism, GM or not, has the potential to cause detrimental effects on the environment, if it has not undergone any safety assessment prior to release. The GMAC Guidelines have thus been put in place to ensure that scientific and thorough assessment is undertaken before any GMOs is released into our environment.
-
Has there been any serious illness or death resulting from eating foods derived from GMOs?
Since the commercialization of the first GM crop nearly two decade ago, there has been no known report of hazards resulting from the consumption of foods derived from such crops.
-
Are there other unpredictable risks from GMOs resulting from our inadequate understanding of genetic modification?
There have been many studies conducted in determining potential negative effects of genetically modified organisms/foods.
To give two examples:
The first study claimed that pollen from pest-resistant Bt corn could kill the larvae of Monarch butterfly.
In the second study, the researchers claimed that autoimmune suppression was observed in rats fed with genetically modified potatoes.
It was found that the two studies cited above were carried out under conditions that do not reflect the actual situation in the field. Peer reviews of these studies have highlighted several flaws in the experiment designs and results analysis.
GMAC fully appreciates that genetic modification is a relatively new technology and that it can potentially harbour certain risks if proper regulation is not in place. The same can be said for all other new and powerful technologies. Scientists and regulators have the responsibility to ensure that risks be brought to the minimum. In this regard, GMAC monitors closely new scientific developments on GMOs and we will continue to work with the regulatory authorities to ensure the protection of consumers and the environment.
-
What are the worst case scenarios of the damaging effects of GMOs on the environment and human population? Do we have contingency plans to cope with such events?
The most likely scenario is the accidental release of GMOs. However, the risk can be minimized significantly if proper containment measures are in place.
Before experiments on GMOs are conducted, risk assessment must be performed to ensure that such these GMOs exert minimal impact even if they should be accidentally released.
In addition, safety control measures to contain the spread of any accidentally released GMOs are in existence and these are required as pre-conditions before experiments on GMOs can be allowed to commence.
-
How do we manage GMOs after their release to the environment?
GMOs released to the environment must be closely monitored. The GMAC guidelines require information on all released GMOs to be collected continually. Any new information regarding potential risks to the environment or to human health must be reported immediately to GMAC and the relevant regulatory authorities.
The regulatory authorities also reserve the right to recall any GMOs approved for release based on its assessment of any new information.
-
What further developments can be expected in the area of GMOs?
While genetic modification has been well established and studied since XXXX, it presents its fair share of uncertainties Whilst that is the case, its potential in various fields such as medicine and agriculture, is simply too valuable to ignore. Credible, science-based decisions will help in resolving some of the controversies and concerns surrounding the technology.
The wide array of GMOs in development at this juncture includes plants with improved disease or climate resistance and food crops with increased nutrient levels.
Other GMOs to be made available in the future may likely include fish species with enhanced growth characteristics, and plants or animals producing pharmaceutically important proteins such as vaccines.